Written by : Dr. Aishwarya Sarthe
July 4, 2024

Per the company, this device is the first in India to secure approvals from the Bureau of Indian Standards (BIS), the Atomic Energy Regulatory Board (AERB), and the Central Drugs Standard Control Organization (CDSCO).
Chennai-based Trivitron Healthcare, a global medical technology firm, has launched its advanced CT scanner, named "Terrene."
The company claims the device to be the first in India to secure approvals from the Bureau of Indian Standards (BIS), the Atomic Energy Regulatory Board (AERB), and the Central Drugs Standard Control Organization (CDSCO).
Sharing thoughts, Dr GSK Velu, CMD of Trivitron Healthcare, said, "The widespread accessibility of healthcare is essential for a stronger India. Our new Terrene CT technology proudly made in India, will revolutionize diagnostics nationwide. We are dedicated to advancing healthcare in India and are excited to introduce more innovations that will benefit every corner of the nation."
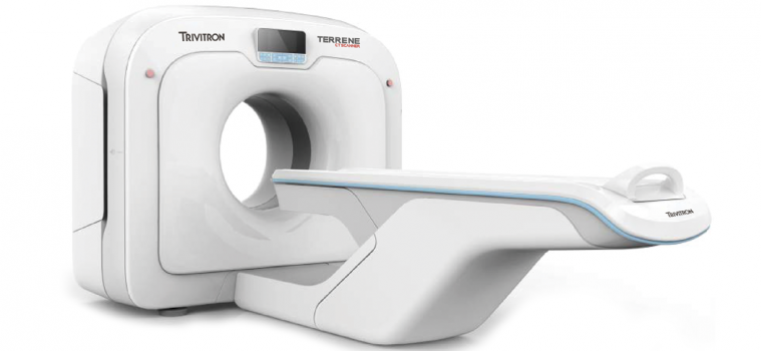
The Terrene CT scanner aims to enhance medical imaging in India with its advanced technology. The scanner boasts improved image quality, reduced radiation dose, and streamlined workflow.
Equipped with sophisticated software algorithms and an intuitive interface, the CT scanner enables healthcare professionals to make accurate diagnoses and provide optimal patient care.
Per the company, Terrene has undergone rigorous testing to meet stringent regulatory standards, securing its approvals from BIS, AERB, and CDSCO.
The device is manufactured at Trivitron's ISO 9001 and ISO 13485 certified facility in the Andhra Pradesh MedTech Zone (AMTZ), Visakhapatnam.
“The introduction of the Terrene CT scanner will be a game changer in the Indian healthcare scenario. We are proud to introduce Terrene, which symbolizes our commitment to speaking the language of care. This milestone achievement of obtaining BIS and AERB approvals highlights our unwavering dedication to innovation and our mission to improve lives" Chandra Ganjoo, group CEO of Trivitron Healthcare said.
In a related development, in March 2022, Trivitron Healthcare acquired a 100% shareholding in US-based Kennedy Company, a manufacturer of radiation protection X-ray shielding material and acoustic barrier products. This acquisition has further strengthened Trivitron’s manufacturing presence in the US.
Since 1997, Trivitron has been a key player in medical technology, operating in 180 countries. It boasts 15 certified manufacturing facilities across India, the USA, Finland, Turkey, and China. The company offers services in in-vitro diagnostics, imaging and radiology, radiation protection, newborn screening, critical care, and operating room solutions.